
POTOMAC, MARYLAND – October XX, 2025 – IGC Pharma, Inc. (“IGC Pharma,” “IGC,” or the “Company”) (NYSE American: IGC), a clinical-stage biotechnology company leveraging AI to develop innovative treatments for Alzheimer’s disease, today announced advances in MINT-AD, the Multimodal Interpretable Transformer for Alzheimer’s. AI tools in Alzheimer’s research by Lleveraging data from the Health and Retirement Study (HRS) International Family of Studies, to IGC Pharma aims to identify unknown socioeconomic risk factors and their interactions influencing aging and cognitive decline. This initiative is part of IGC Pharma’s broader mission to revolutionize Alzheimer’s treatment with precision medicine and AI-driven insights.

Among the datasets used in MINT-AD training, IGC Pharma is leveraging 14 large-scale longitudinal aging and cognition studies spanning the USA, Africa, Mexico, China, Costa Rica, Puerto Rico, Chile, Brazil, England, Thailand, Indonesia, India, and Korea, all adhering to HRS protocol. This approach enables AI to identify critical socioeconomic factors contributing to early cognitive decline across populations with diverse sociocultural contexts, offering a new way to predict, stratify, and intervene before Alzheimer’s takes hold.

“Understanding the socioeconomic factors that contribute to cognitive decline is critical to advancing more effective Alzheimer’s treatments, at a time when it is increasingly becoming critical to improve healthcare strategies for the rapidly aging population,” said Ram Mukunda, CEO of IGC Pharma. “By leveraging AI and global longitudinal datasets, we are unlocking identifying hidden relationships between socioeconomic factors and cognitive health, allowing us to develop personalized non-pharmacological interventions that could transform public health policies and improve clinical outcomes. This research complements our ongoing IGC-AD1 clinical trials, where we are developing targeted therapies for both agitation in Alzheimer’s dementia and potential disease-modifying treatments.“

“This quarter reflects the disciplined execution of our dual strategy in clinical trials and artificial intelligence, positioning IGC to create meaningful shareholder value,” said Ram Mukunda, CEO of IGC Pharma. “We’ve accelerated our lead Alzheimer’s program past the 50% enrollment mark, demonstrating our operational focus. Simultaneously, we converted a previously cash-negative manufacturing facility into a favorable contract that strengthens our balance sheet. The NIA award underscores that our unique AI platform is not just a concept, but a recognized tool accelerating Alzheimer’s diagnostics. We are confident this progress sets the stage for rapid advancement in the quarters ahead.”

Operational & Pipeline Progress in Detail:
Phase 2 CALMA Trial Expansion: The ongoing Phase 2 CALMA trial for IGC-AD1 was strategically expanded to new international and domestic sites, including Island Health’s Royal Jubilee Hospital in Victoria, British Columbia, Canada, and Lynn Health Science Institute (LHSI) in Oklahoma City, Oklahoma. This expansion is designed to accelerate the remaining enrollment and enhance patient diversity.
Patent Protection Secured: The Company received a Notice of Allowance from the USPTO for its patent application IGC510 (US 17/613,909), a proprietary method for treating individuals suffering from stammering, stuttering, or Tourette’s syndrome.

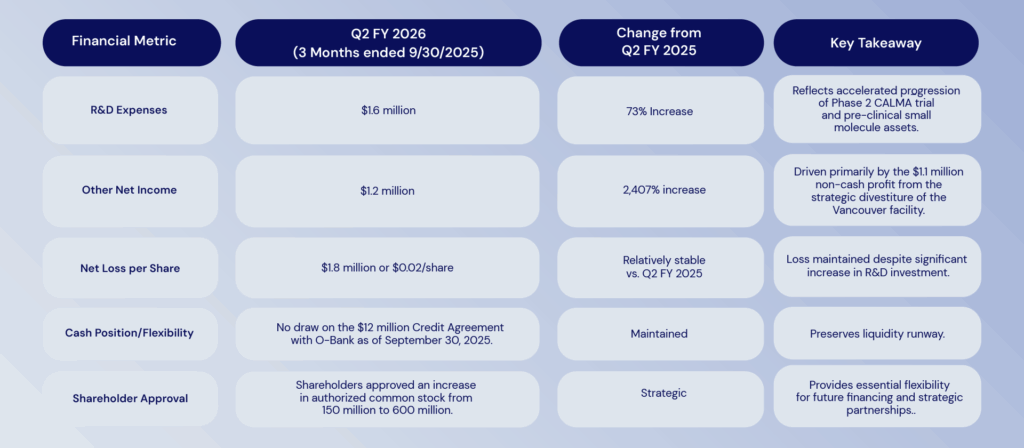
Financial Summary:
IGC expects continued strong momentum heading into the second half of fiscal 2026, with key strategic priorities focused on pipeline execution and AI commercialization:
-
Clinical Acceleration: Completing patient enrollment in the CALMA Phase 2 study.
-
AI Monetization: Initiating key collaborations for piloting MINT-AD (IGC’s AI diagnostic platform) with academic and clinical partners.
-
Pipeline Deepening: Advancing the promising IGC-M3 preclinical asset.
-
Strategic Partnerships: Evaluating strategic partnerships across AI diagnostics and Alzheimer’s therapeutics.
-
Financial Stewardship: Strengthening operational efficiency while preserving cash runway and financial flexibility.
IGC Pharma’s financial statements for the quarter ended September 30, 2025 filed on Form 10-© are available on www.sec.gov.
About IGC Pharma (dba IGC):
IGC Pharma (NYSE American: IGC) is a clinical-stage biotechnology company leveraging AI to develop innovative treatments for Alzheimer’s and metabolic disorders. Our lead asset, IGC-AD1, is a cannabinoid-based therapy currently in a Phase 2 trial (CALMA) for agitation in Alzheimer’s dementia. Our pipeline includes TGR-63, targeting amyloid plaques, and early-stage programs focused on neurodegeneration, tau proteins, and metabolic dysfunctions. We integrate AI to accelerate drug discovery, optimize clinical trials, and enhance patient targeting. With a complete patent portfolio and a commitment to innovation, IGC Pharma is advancing breakthrough therapies.
Forward-Looking Statements:
This press release contains forward-looking statements. These forward-looking statements are based largely on IGC Pharma’s expectations and are subject to several risks and uncertainties, certain of which are beyond IGC Pharma’s control. Actual results could differ materially from these forward-looking statements as a result of, among other factors, the Company’s failure or inability to commercialize one or more of the Company’s products or technologies, including the products or formulations described in this release, or failure to obtain regulatory approval for the products or formulations, where required, or government regulations affecting AI or the AI algorithms not working as intended or producing accurate predictions; general economic conditions that are less favorable than expected; the FDA’s general position regarding cannabis- and hemp-based products; and other factors, many of which are discussed in IGC Pharma’s U.S. Securities and Exchange Commission (“SEC”) filings. IGC incorporates by reference its Annual Report on Form 10-K filed with the SEC on June 27, 2025, as if fully incorporated and restated herein. Considering these risks and uncertainties, there can be no assurance that the forward-looking information contained in this release will occur. IGC Pharma, Inc. assumes no obligation to update forward-looking statements contained in this release as the result of new information or future events or developments.
Contact Information:
Rosalyn Christian / John Nesbett
IMS Investor Relations
igc@imsinvestorrelations.com
(203) 972-9200






